The year is off to an eventful start, and we’re excited to share what we’ve been working on. Here’s what you can read more about in this newsletter:
- What’s Happening at Friends?: Catch up on the highlights from our recent advocate-focused webinar, where a diverse panel of experts including voices from advocacy, research, and policy discussed the future of diagnostics in oncology and the vital role patients play in supporting innovation.
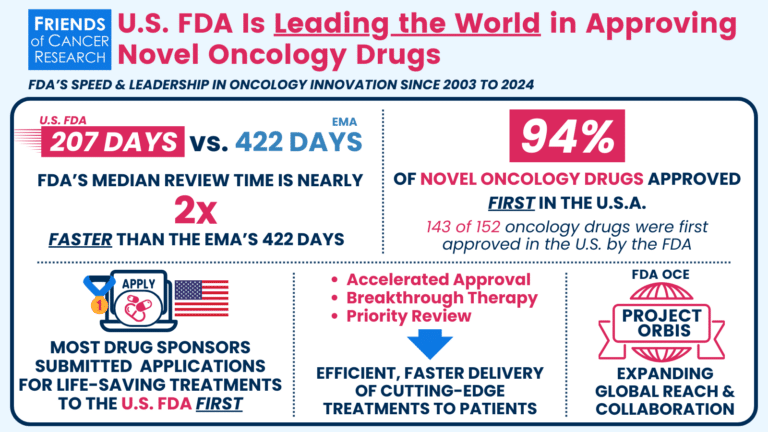
- Did You Know?: The U.S. Food and Drug Administration (FDA) is a global leader in getting innovative treatments to patients with cancer. Read our updated analysis comparing 20 years of new cancer drug approvals by FDA and European Medicines Agency (EMA).
- Mark Your Calendars: Friends is hosting a public meeting on May 9th focused on next-generation therapies. Be sure to register for the advocate recap event on May 21st for additional opportunities to engage with experts!
- Important News In Cancer Research: Recent shifting policy priorities and changes across the federal government could impact cancer research and care. Read Friends’ perspective on these changes and their impacts.
What's Happening at Friends?
On February 19th, Friends convened a webinar to engage with patient advocates on advancing diagnostics and regulatory innovations. The discussion built on topics from the February 4th public meeting, delving into the significance of emerging technologies like AI-enabled diagnostics and digital pathology, as well as the challenges of developing diagnostic tests for rare biomarkers. The Q&A session sparked discussions on several key topics, including:
- Clinical Implications of AI-enabled Diagnostics: Panelists reiterated AI and digital pathology have the potential to reduce misdiagnoses and improve the precision of cancer diagnostics, leading to better treatment decisions. The discussion also highlighted how harmonization and use of these tools across clinical settings can help reduce disparities in care and ensure all patients receive the most effective treatments based on their specific cancer type.
- Patient Advocacy in Research: The webinar underscored the critical role of patient advocates in shaping research priorities and ensuring that diagnostic innovations address real-world patient needs.
- Collaborative Approach: Panelists stressed the need for stronger collaboration between advocates, drug developers, and regulators to streamline the development and validation of new diagnostic tools. We all have a role – advocates amplify patient needs, drug developers drive innovation, and regulators establish clear and innovative pathways to expand patient access. As the landscape evolves, multidisciplinary collaborations are more critical than ever to accelerate progress and deliver meaningful advancements to patients.
- Ethical Considerations: The discussion highlighted the ethical implications of biopsies in clinical trials and importance of patient data protections when developing AI-enabled diagnostics. These conversations reiterated the need for clear communication and informed consent.
Missed the event? Explore how AI can enhance cancer diagnostics and where your voice can make an impact.
A big thank you to our panelists for a great discussion!
Megan Doyle
Associate Vice President, Assistant General Counsel, Eli Lilly & Company
Joan Mancuso
Friends Advisory Advocate, Patient Advocate
George Green
Independent consultant at GA Green Consulting LLC working with the pharma and biotech industries to develop Precision Medicine and Companion Diagnostic products.
Alain Silk
Senior Director of Regulatory Affairs, Tempus AI
Please join us for our advocate recap webinar on May 21st – we’ll be discussing how new manufacturing approaches for complex therapies can enhance patient-centered care and improve access to these transformative medicines.
Did You Know?
Our recent analysis spanning two decades of novel cancer drug approvals reaffirms that the FDA continues to expedite access to innovative treatments, outpacing its European counterpart (the European Medicines Agency [EMA]) in approval timelines. The FDA’s efficiency and innovative regulatory approaches ensure that patients in the U.S. benefit from life-saving therapies sooner, driving progress in cancer care.
Mark Your Calendars
⬇️ = Reduced Advocate Registration | 🟩 = Free | 🔷 = Friends’ Event
🔷🟩 April 7, 2025 | Biopharma Congress: Ready for a New Era |
| ⬇️ April 25 – 30, 2025 | 2025 American Association for Cancer Research (AACR) Annual Meeting |
| 🟩 May 5 – 8, 2025 | 13th Annual Symposium on Global Cancer Research (ASGCR) |
| 🔷🟩 May 9, 2025 | Unlocking Next-Generation Therapies |
| 🔷🟩 May 21, 2025 | Unlocking Next-Generation Therapies Meeting Recap: Sharing Next Steps and Opportunities with Advocates |
| ⬇️ May 30 – June 3, 2025 | 2025 American Society of Clinical Oncology (ASCO) Annual Meeting ⭐️ Advocates can attend ASCO virtually for free, or in-person for a reduced rate, more info here. |
| ⬇️ June 15 – 19, 2025 | DIA 2025 Global Annual Meeting ⭐️ DIA scholarship opportunity for advocates here. |
Important News in Cancer Research
The Trump administration’s recent actions have largely focused on restructuring federal agencies, reducing the federal workforce, and reassessing policy and budget priorities. These changes impact various sectors, including those that drive progress in cancer research and care. Agencies such as the FDA and National Institutes of Health (NIH) are among those affected, medical innovation, research funding priorities, and public health programs that patients rely on. As these developments continue, stakeholders, such as Friends, continue to monitor the potential impact on research, public health initiatives, and long-term policy objectives.
To read more on Friends’ perspective on these changes click here.
Read more cancer research news on Friends of Cancer Research’s website.
ProgressforPatients.org
Interested in learning more about the drug development and how U.S. FDA functions?
Take the free self-paced ProgressforPatients.org online course!